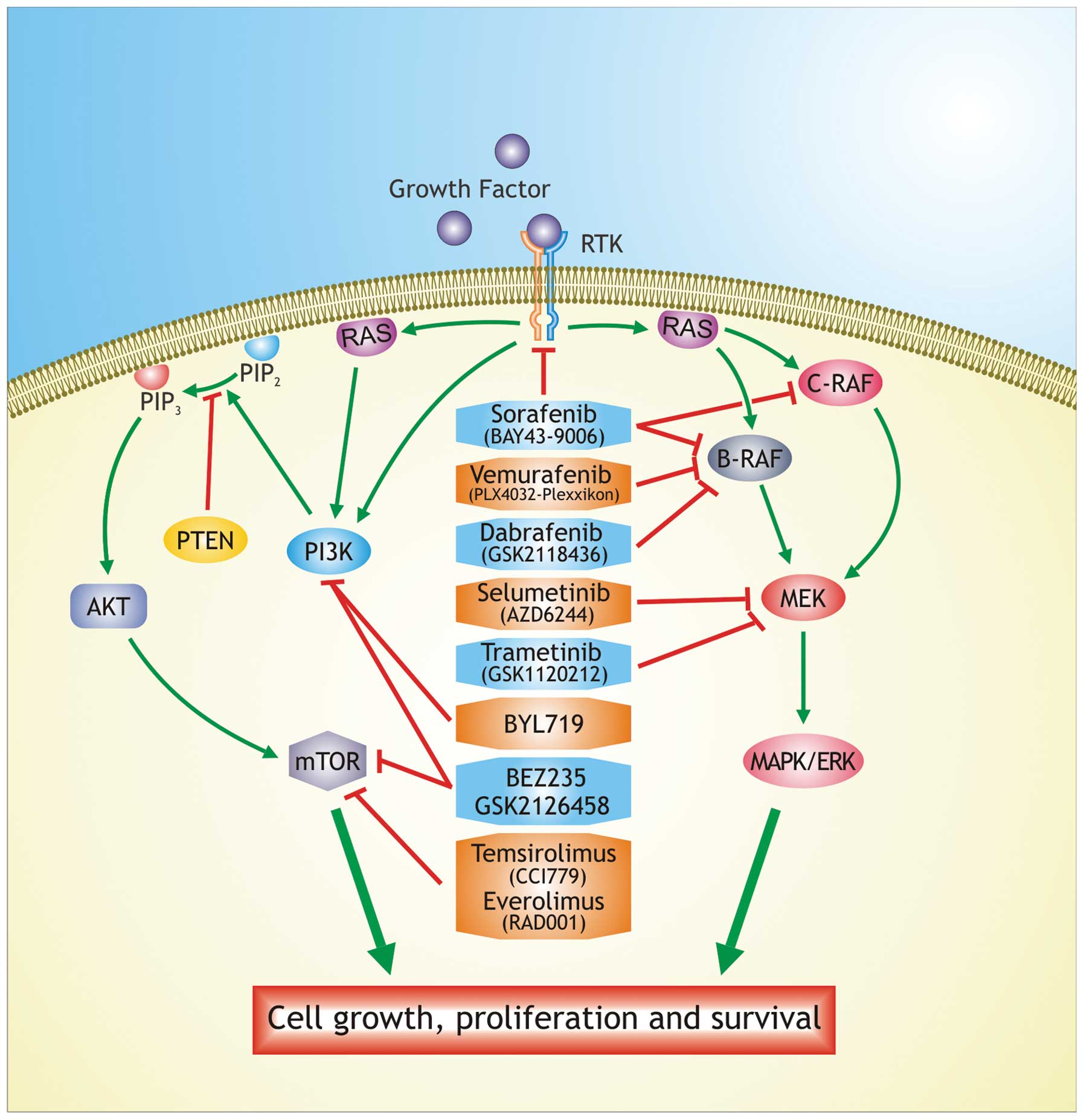
Melanoma (Emerging Cancer Therapeutics)
PI3K and MEK inhibitor combinations are well tolerated and can be administered at therapeutic doses; however, additional studies are required to establish the precise tumor properties that will better respond to therapy Rapamycin is a mTORC1 blocker of the first generation, while everolimus RAD and temsirolimus CCI are considered agents of second generation, which allosterically inhibit the mTOR complex 79 , 80 ; these agents do not have high specificity in targeting melanoma tumor cells New therapeutic approaches involve the use of immunotherapy for the treatment of cancer.
Immunotherapy is based on increasing the immune defenses to eliminate the cancer cells to gain chemotherapeutic effect, and aiming to arrest the cell cycle inducing apoptosis Immunotherapy may be used for tumors because they express tumor associated antigens 82 , 83 ; melanoma lesions often contain a high number of infiltrative T-cells specific to melanocyte tumor-associated antigens such as MART1, gp and tyrosinase An approach to eliminate the melanoma cells is to increase the natural function of these cytotoxic T lymphocytes CTL The first immunotherapy to be approved by the Food and Drug Administration FDA for treatment of advanced melanoma was interleukin-2 IL-2 but, like dacarbazine, response rates were low even at high-doses of treatment Its use in clinical practice is limited by the severe toxic side-effects 88 — This immunotherapeutic augments the antitumor T-cell response resulting in uncontrolled T-cell proliferation and for this reason is associated with a substantial risk of immune-related adverse reactions 91 , Ipilimumab acts by an indirect mechanism through T-cell mediated antitumor immune responses.
The most common severe immune-mediated adverse reactions are enterocolitis, hepatitis, dermatitis, neuropathy and endocrinopathy; these reactions can occur both during the treatment, or weeks or months after discontinuation of treatment 93 , In a clinical study, untreated patients with advanced melanoma received a higher dose of ipilimumab with or without dacarbazine or dacarbazine plus placebo.
Patients treated with dacarbazine and ipilimumab showed a significant increase of the overall survival rate compared with those treated with dacarbazine plus placebo The treatment with ipilimumab in advanced melanoma patients was also considered in concomitance with the experimental vaccine glycoprotein gp Pre-treated patients were randomized for the administration of ipilimumab alone or in combination with gp or gp alone.
It was shown that the combination with ipilimumab and gp did not improve survival when compared with ipilimumab alone, suggesting that ipilimumab remains the treatment with most efficacy for advanced melanoma Besides ipilimumab, also nivolumab, a monoclonal antibody directed against the PD-1 receptor or its ligand PD-L1 has been reported Nivolumab has been administered as monotherapy; most recent data suggest that nivolumab and ipilimumab can be administered concomitantly with a manageable safety profile Immunotherapy is becoming an important support to melanoma treatment Resistance to therapeutic agents, both chemical or biological agents, remains the main problem in the management of the therapy in melanoma.
This combination reduces the skin toxicities and may also enhance the antitumoral effects by synergistically suppressing ERK pathways activity In a phase I study the effectiveness of vemurafenib was tested in combination with an inhibitor of MEK showing a tumor reduction in melanoma patients, while in a phase III trial vemurafenib alone was compared with vemurafenib in combination with MEK inhibitor This combined therapy may be able to overcome the resistance mechanisms leading to apoptosis.
These combinations appear well tolerated and can be administered as therapeutic doses The approval of ipilimumab represents a further treatment option for melanoma patients. The National Comprehensive Cancer Network NCCN now lists ipilimumab and vemurafenib among the small number of preferred systemic regimens for treating advanced and metastatic melanoma The combination of vemurafenib with immunotherapy could overcome the resistance mechanisms because immunotherapy drugs have low response rates but relatively long durations of response in a large subset of responding patients, by contrast, B-RAF inhibitors have high initial response rates but rarely produce long-term durable responses 95 , Ipilimumab targets the tumors indirectly by activation of the immune system therefore it is likely to be efficacious in melanoma patients with and without the B-RAF VE mutation Some clinical trials have shown that MAPK pathway inhibition with a selective inhibitor of B-RAF VE increase expression of melanoma-derived antigens by the tumor and increased the recognition of melanoma cells by antigen-specific T cells and, selective inhibition did not have deleterious effects on T cell proliferation or function , B-RAF inhibitor treatment led to increased number of tumor infiltrating lymphocytes in tumor biopsies obtained 10—14 days after treatment initiation.
This increase was associated with a reduction in tumor size and an increase in necrosis in on-treatment biopsies , These results suggested that at least a subset of patients might be able to receive treatment with curative intent with interleukin-2 or ipilimumab without compromising their ability to benefit from B-RAF inhibitor treatment if they fail to achieve a durable response Finally, the effects of the new nitric oxide NO donating compound S,R phenyl-4,5-dihydroisoxazole acetic acid-nitric oxide GITNO on the A human melanoma cell line were investigated by our group.
Cancer research is converging on understanding the roles of signal transduction pathways in drug resistance and sensitivity. Targeting various effectors of these pathways with pharmacologic inhibitors may arrest melanoma cell proliferation. The use of MAPK and AKT inhibitors for the treatment of melanoma indicates that the response rate of these new molecular targeted agents is higher compared to the standard chemotherapy.
However, additional studies are needed to better define the mechanisms of resistance to these novel biological therapies. National Center for Biotechnology Information , U. Published online Jun 3. Author information Article notes Copyright and License information Disclaimer.
Received May 27; Accepted Jun 3. The article may be redistributed, reproduced, and reused for non-commercial purposes, provided the original source is properly cited. This article has been cited by other articles in PMC. Abstract Cutaneous melanoma is an aggressive cancer with a poor prognosis for patients with advanced disease. Introduction Cutaneous melanoma, is a form of aggressive cancer that develops from melanocytes. Open in a separate window. Immunotherapy and ipilimumab New therapeutic approaches involve the use of immunotherapy for the treatment of cancer.
Combination therapy Resistance to therapeutic agents, both chemical or biological agents, remains the main problem in the management of the therapy in melanoma. Conclusion Cancer research is converging on understanding the roles of signal transduction pathways in drug resistance and sensitivity. Exposure to the sun and sunbeds and the risk of cutaneous melanoma in the UK: A landscape of driver mutations in melanoma.
Cancer Epidemiol Biomarkers Prev. Mutations of the BRAF gene in human cancer. Analysis of BRAF mutation in primary and metastatic melanoma. Garnett MJ, Marais R. B-RAF is a human oncogene. Plasticity of the cancer cell: Gene therapy for advanced melanoma: B-RAF is a therapeutic target in melanoma. Final report of the phase I clinical program of the novel raf kinase inhibitor BAY in patients with refractory solid tumors. Preclinical and clinical development of the oral multikinase inhibitor sorafenib in cancer treatment. Sorafenib in advanced melanoma: Molecular targeted therapies in metastatic melanoma.
Sorafenib and dacarbazine as first-line therapy for advanced melanoma: Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Jang S, Atkins MB. Which drug, and when, for patients with BRAF-mutant melanoma? N Engl J Med. RAS mutations are associated with the development of cutaneous squamous cell tumors in patients treated with RAF inhibitors.
Inhibition of mutated, activated BRAF in metastatic melanoma. Shao Y, Aplin AE. Akt3-mediated resistance to apoptosis in B-RAF-targeted melanoma cells. Mechanisms of resistance to RAF inhibitors in melanoma. Pharmacodynamic effects and mechanisms of resistance to vemurafenib in patients with metastatic melanoma.
Dabrafenib in BRAF-mutated metastatic melanoma: Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: Pigm Cell Melanoma R. Melanoma biology and new targeted therapy. Update on the targeted therapy of melanoma. Curr Treat Options Oncol. Chang L, Karin M. Mammalian MAP kinase signalling cascades. Thompson N, Lyons J. Activity of the oral MEK inhibitor trametinib in patients with advanced melanoma: GSK JTP is an inhibitor of MEK activity and activation with favorable pharmacokinetic properties for sustained in vivo pathway inhibition.
MEK and the inhibitors: Advances in personalized targeted treatment of metastatic melanoma and non-invasive tumor monitoring. The oncogenic properties of mutant palpha and pbeta phosphatidylinositol 3-kinases in human mammary epithelial cells. The drawback is that the procedure requires a solid tumor that is surgically reachable. The tumor cell need not be autologous, allogeneic tumor cell vaccines present the possibility of an even wider array of antigen presentation and are easier to produce.
The use of various different adjuvants have been applied to this method, LPS can be included to stimulate the immune response, or tumor cells can be genetically engineered to express cytokines that promote proliferation of immune cells such as GM-CSF, IL-2, and Ftl3 Isolated from Mycobacterium bovis , BCG has been used decades worldwide as a vaccine against tuberculosis By the s, it was discovered that administration of BCG alone into the tumor environment was an effective treatment for bladder cancer; the interaction between the pathogen-associated molecular patterns and lymphocyte pattern recognition receptors prime an immune response that can then act upon the tumor.
Several whole-cell tumor vaccines have entered clinical trials but, representative of the cancer vaccine field as a whole, results were often not as promising as hoped. Canvaxin was an allogeneic tumor cell vaccine that entered phase III clinical trials in for treatment of melanoma, after failing to show a survival benefit the trials were ended early and the vaccine was abandoned These trials also reported a lack of autoimmune toxicities, implying that the safety of vaccines could make them an attractive option in the future.
DNA vaccines contain the requisite TAAs encoded onto a bacterial plasmid which is injected into the patient. The plasmid is up-taken by local cells, including APCs which naturally move to the site of the injection, once up-taken, the APCs can then directly produce their own antigen. While conceptually promising, DNA vaccines have yet to show benefit when tested in clinical trials. Alternatively, the genetic info can be in the form of RNA, which undergoes rapid enzymatic degradation and therefore avoids any possible toxicity.
RNA vaccines are also currently in clinical trials. The TAAs used can vary and one vaccine can contain multiple antigens. Applications have been found for breast, testicular, and prostate cancer, as well as melanoma. While easier to manufacture than whole-cell vaccines, peptide vaccines present a much smaller array of antigens to the host immune system.
- Apollo and Americas Moon Landing Program: Apollo 17 Official NASA Mission Reports and Press Kit - 1972 Sixth and Final Lunar Landing - Astronauts Cernan, Evans, and Schmitt!
- Modeling Fragile X Syndrome: 54 (Results and Problems in Cell Differentiation)!
- jankenasobi (Japanese Edition).
- Appointment with a Manifestation?
- Emerging targeted therapies for melanoma treatment (Review).
However, upon employing genetic modification, the peptide sequence of epitopes in these vaccines can be modified to enhance antigenicity and therefore promote binding to T lymphocytes. The gp vaccine for the treatment of metastatic melanoma is an example of such and one of the more researched peptide vaccines to date.
The protein gp is a transmembrane glycoprotein and features an amino acid substitution in the peptide sequence for enhanced lymphocyte binding. Gp has reached phase III clinical trials but results have been mixed, those studies showing therapeutic benefit have not been reliably produced, and in most trials it is used in combination with another I-O therapy such as IL-2 therapy or immune checkpoint inhibitors to provide a patient benefit Adjuvants for these vaccines are similar to those used for the whole-cell vaccines such as BCG and LPS; bacterial or viral antigens seem to be the most effective at eliciting an immune response.
Viruses are naturally potent at stimulating an immune response, making them ideal not just as adjuvants but as the complete vector for the vaccine. TAAs are encoded into an attenuated virus, which will then transduce host cells and lead to expression of the antigen; among the transgenes, IL-2 and GM-CSF can also be encoded as in previously mentioned vaccines to increase lymphocyte proliferation.
The high replication rate of viruses compared to living cells provides an advantage as a vector. Members of the poxviridae family poxviruses are the most popular because they can accommodate large amounts of genetic info, are stable, and replicate with accuracy Attenuation occurs through deletion of pathogenic genes, or in the case of the MVA vaccine modified vaccinia virus , numerous in vivo serial passages resulting in a weakened viral genome 56 ; either method produces a strain that can infect cells and cause protein expression but cannot damage the host. Modified vaccinia ankara vaccine has the advantage of being safe for use in immunocompromised patients.
Gene expression using poxviruses can occur for about 1—3 weeks and occurs without the risk of insertional mutagenesis. Poxvirus vectors are used as the priming vaccine but due to the adaptive host immune response, will not provide further benefit with repeat administration, therefore, boosters are given using avipoxvirus vectors, which do not elicit the production of neutralizing antibodies in mammals and allow for the continuation of therapy.
Viral vector technology has been extended even further with TRICOM, a formulation of transgenes for the treatment of prostate cancer that combines prostate-specific antigen and three different T-cell costimulatory molecules to enhance the immune response. Although failing to achieve clinical endpoints, the treatment arm did see a significant improvement in OS A phase II trial published in combined TG and cytokine therapy for treatment of renal carcinoma.
Results showed an improved OS but no clinical response The use of viruses in I-O has been taken one step further by using them not as the vector for therapy but as the drug therapy agent itself. These OVs are engineered to selectively infect and kill tumor cells without the pathogenicity to healthy cells seen in a normal virus. Figure 3 The idea for using viruses as a cancer treatment has existed for a few decades but only now has become feasible due to advances in genetic technology.
Production of an OV focuses on safety, adherence to quality and purity, and achievement of a high enough titer. Herpes simplex viruses HSV , adenoviruses, measles viruses, reoviruses, vaccinia viruses, and more have all been utilized as treatments. Like the aforementioned viral vectors, OVs undergo deletion of genes to reduce their pathogenicity to healthy cells while preserving their ability to replicate in vivo.
An example is the deletion of the viral gene encoding thymidine kinase in HSV. This enzyme is critical for DNA synthesis and is expressed by the virus but also in proliferating human cells. Deletion of the gene in the virus makes it dependent on the host enzyme to replicate and therefore causes it to preferentially target rapidly proliferating tumor cells Selectivity is further enhanced by modifying viral attachment proteins to target TCRs.
Modifying the viral genome to make a virus able to replicate only in rapidly proliferating cells is the main method of engineering these viruses to selectively target the tumor. Once the genetic modification of the virus is complete, manufacture is a meticulous stepwise process.
Growing the cells presents the first hurdle, separating the virus from the supernatant is a delicate process and therefore cells are ideally grown serum-free to avoid involving additional components that would complicate purification; conversely, this is obviously not the most efficient method of cell culture. After infection and incubation, a lysis buffer is added followed by a nuclease that degrades nucleic acids of the producer cell.
Viral particles are then purified from the lysate through processes that can vary depending on the characteristics of the virus size, stability under reagents, heat, physical stress, etc. Methods include centrifugation, ion-exchange chromatography, size exclusion chromatography, tangential flow filtration, and more. Once purified, the virus is tested for contaminants, potency, and identity through methods like PCR or Western blotting Direct tumor cell cytotoxicity caused by the virus is often a result of the activation of apoptotic pathways, including Ras and caspase signaling. Virus-infected tumor cells also express MHC-1 marking them for cell lysis just as in a natural viral infection of healthy cells.
This challenge was mentioned earlier in regards to virus vectors and is even more prevalent in the use of OVs. The virus is at risk of being eliminated by the innate immune response before eliciting any effect on the tumor; consequently, the route of administration is key in this therapy with intratumoral being preferred over IV. The development of an adaptive immune response toward viral antigens also eliminates the possibility of continuous treatment, which is critical in any oncologic therapy.
However, the immune memory is at the same time key to eliminating the tumor; use of a parvovirus OV in mice infected with GL glioma achieved a complete response that was also immune to rechallenge with xenografted glioma cells, and modified VSV was also shown to induce tumor immunity in B16 melanoma infected mice It is critical therefore that the virus be able to avoid attacking and downregulating host lymphocytes, even though it is possible they would in turn reduce efficacy of the OV.
The prime and boost method is utilized yet again with sequential administration of two OVs of different species to circumvent this issue. The activity of one virus might also not compliment another, the resulting cytokine release of initial therapy could hinder the efficacy of the following OV treatment. This raises the question of how to combine OVs with existing mainstays of cancer therapy, whether they prime or inhibit the immune system.
Currently there are only two OVs that have been brought to market: The median OS was also significantly improved by over 4 months An advantage to T-Vec and OV therapy at large is that it is well tolerated with minimal and low grade side effects. Oncorine is a modified adenovirus approved in China for head, neck, and esophageal cancer in It contains a deletion of the E1B gene responsible for inactivating p53 to allow continued viral replication, this makes the virus selective for tumor cells which inactive p53 on their own.
A Chinese study published in compared Oncorine with or without transhepatic arterial chemoembolization therapy in patients with hepatocellular carcinoma. The study showed a significant increase in complete response Adverse events were similar between the two groups and were low grade and reversible, such as fever, pain, and elevated white blood cells. Naturally occurring OVs without any genetic modification have also shown potential as cancer therapeutics. Reolysin is a wild type strain of reovirus that achieved FDA orphan drug designation in , it targets tumors through its natural selectivity for cells with over-activation of the Ras pathway.
A recent phase III trial has shown Reolysin to improve OS in patients with recurrent head and neck cancer when combined with a combination chemotherapy regimen compared to chemotherapy alone A BiTE is a bispecific antibody featuring the minimal binding domains of the Fab antibody portion called the single-chain fragment variables of two antibodies linked via a non-immunogenic, 5-amino acid repetitive linker The linkage forces the formation of an immunological synapse Figure 4 , where the T-cell then perforates the tumor cell membrane and releases granzymes that induce a caspase-mediated apoptosis 69 , in addition to cytokine release and T-cell proliferation.
Construction of a bispecific T-cell engagers BiTE is shown in the lower part of the diagram. The single chain fragment variables Fab-binding domains of the two desired antibodies are joined via amino acid linkage. The linkage forces the formation of an immunological synapse by drawing the two together, causing recognition of the TAA and activation of the T-cell.
OS and RFS were 6. CRS has been observed in other trials and seems to correlate with disease burden 70 , steroid pretreatment with dexamethasone has been identified as an effective manner of controlling CRS. The adverse events observed were all dose dependent and resolved upon the discontinuation of treatment The drug also features a small protein size at 55 kDa less than half that of a monoclonal antibody and rapid clearance, such clearance necessitates a continuous infusion but could also speak for its ability to easily reach the site of action and becomes an asset when needing to quickly reverse toxicities.
Bispecific T-cell engager therapy is currently being expanded to the treatment of solid tumors, under investigation are agents targeting CEA, prostate-specific membrane antigen, and epithelial cell adhesion molecule EpCAM. An in vitro study using it to treatment uterine and ovarian carcinosarcoma cell lines showed an increase in T-cell cytotoxicity from 1.
Monitoring patient response to immune checkpoint inhibitors can in some cases be challenging when treatment results in non-conventional response kinetics, contradictory to those which would be expected in conventional therapies. Responses can vary, some tumors will show the expected immediate response or lack of progression, but others will exhibit a preliminary progression of the tumor that is then followed by responsive or stable disease.
This has been called pseudoprogression and occurs as a result of the newly activated T-cells infiltrating the tumor causing what appears radiographically as flaring and progression of the lesion. This necessitates careful timing in the assessment of tumor response to checkpoint inhibitors. For example, it has been recommended that the initial assessments of ipilimumab do not begin until 12 weeks following the start of therapy It also becomes the burden of physicians to differentiate what could be pseudoprogression from what is true tumor progression.

As a general rule, pseudoprogression involves new or progressing lesions without any associated response by the patient or worsening of symptoms, in some cases the increased T-cell infiltration can also be confirmed by tumor biopsy. A set of response criteria specific to I-O therapies were published in based on the findings by field experts at that time. It is meant to be used as an addendum to the standard response evaluation criteria in solid tumors RECIST for assessing therapeutic outcomes in cancer.
The four immune-related response criteria irRC identified to correlate with positive outcomes are as follows: The impact of using these adjusted criteria was shown in a study, where the treatment of advanced melanoma patients with Pembrolizumab was evaluated using both irRC and RECIST v1. Novel means of assessment in addition to the new criteria are also still being explored, the unique interaction between I-O therapy and the tumor and its environment make RECIST methods such as measurement of the tumor lesions unreliable.
There are currently several clinical trials ongoing evaluating the use of new I-O imaging methods; many include radioligands detectable by PET scanning engineered to target receptors expressed on activated TILs, MDSCs, and checkpoint receptors. ACT therapy offers a unique approach where the genetically modified T-cell is also transfected with a gene that will allow for detection of the marker 72 , this is something that could potentially be applied to vaccine therapy as well.
The currently approved I-O therapies, although few, are already making an impact in the treatment of cancers of many varieties. Their success and the booming interest in this field at large has led to the development of new therapeutics of all types listed above, some of which are summarized in Table 1.
This is evidence of the strength and promise of this field, perhaps the next approved I-O therapy could be a long-awaited cancer breakthrough. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The treatment of malignant tumors by repeated inoculations of erysipelas: Am J Med Sci Revisiting immunosurveillance and immunostimulation: J Transl Med 3 1: Tumour-specific immunity against spontaneous rat tumours.
Emerging Immunotherapy Options for Malignant Melanoma - Cancer Therapy Advisor
Int J Cancer 1: Selective in vitro growth of T lymphocytes from normal human bone marrows. Hanahan D, Weinberg RA. Cell 5: Nat Biotechnol 32 1: Trastuzumab — mechanism of action and use in clinical practice. N Engl J Med 1: Mechanisms of action of bevacizumab as a component of therapy for metastatic colorectal cancer.
Semin Oncol 33 5 Suppl Buchbinder EI, Desai A. Am J Clin Oncol 39 1: Immunol Rev 1: J Immunol 8: Interaction of the cytoplasmic tail of CTLA-4 CD with a clathrin-associated protein is negatively regulated by tyrosine phosphorylation. Biochemistry 36 Science Ann N Y Acad Sci Lancet Oncol 13 9: Activator protein 1 suppresses antitumor T-cell function via the induction of programmed death 1. Nat Med 16 Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J Exp Med 6: Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation.
Sci Signal 5 J Adv Pract Oncol 6 3: Clinical utility of nivolumab in the treatment of advanced melanoma.
- Emerging targeted therapies for melanoma treatment (Review).
- Drei Welten - drei Leben (German Edition).
- Stuntman!: My Car-Crashing, Plane-Jumping, Bone-Breaking, Death-Defying Hollywood Life.
- Rastreador, El (Spanish Edition)?
- 101 Hilarious Animal Jokes - Brand-New Howlers That Will Have Your Kids Barking With Laughter.
- Special offers and product promotions;
- The Perfect Answer.
Ther Clin Risk Manag Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med Big opportunities for small molecules in immuno-oncology. Nat Rev Drug Discov 14 9: Tadalafil augments tumor specific immunity in patients with head and neck squamous cell carcinoma. Clin Cancer Res 21 1: Arun B, Goss P. The role of COX-2 inhibition in breast cancer treatment and prevention.
Semin Oncol 31 2 Suppl 7: Cyclooxygenase-2 inhibitors in colorectal cancer prevention: Cancer Epidemiol Biomarkers Prev 17 8: Clinical profile of cyclooxygenase-2 inhibitors in treating non-small cell lung cancer: PLoS One 11 3: Cyclooxygenase-2 expression is an independent predictor of poor prognosis in colon cancer.
Clin Cancer Res 14 Mustafa A, Kruger WD. Suppression of tumor formation by a cyclooxygenase-2 inhibitor and a peroxisome proliferator-activated receptor gamma agonist in an in vivo mouse model of spontaneous breast cancer. Imiquimod — its role in the treatment of cutaneous malignancies. Indian J Pharmacol 47 4: Oncoimmunology 5 3: Antitumor activity and safety of combination therapy with the toll-like receptor 9 agonist IMO, erlotinib, and bevacizumab in advanced or metastatic non-small cell lung cancer patients who have progressed following chemotherapy. Cancer Immunol Immunother 63 8: Maintenance treatment with the immunomodulator MGN, a toll-like receptor 9 TLR9 agonist, in patients with metastatic colorectal carcinoma and disease control after chemotherapy: J Cancer Res Clin Oncol 9: A first-in-human, first-in-class, phase I study of carlumab CNTO , a human monoclonal antibody against CC-chemokine ligand 2 in patients with solid tumors.
Melanoma/Skin Cancers
Cancer Chemother Pharmacol 71 4: Transfus Apher Sci 46 3: Adoptive cell transfer as personalized immunotherapy for human cancer. Cancer Immunol Res 3 Oncoimmunology 4 Tumour MHC class I downregulation and immunotherapy review.
Oncol Rep 10 6: Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res 17 Chimeric antigen receptor T cells for sustained remissions in leukemia. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO J Clin Oncol 29 7: J Immunother 36 2: Autoimmunity in a phase I trial of a fully human anti-cytotoxic T-lymphocyte antigen-4 monoclonal antibody with multiple melanoma peptides and montanide ISA 51 for patients with resected stages III and IV melanoma.
J Clin Oncol 23 4: First-in-human phase 1 study of the oral inhibitor of indoleamine 2,3-dioxygenase-1 epacadostat INCB in patients with advanced solid malignancies. Clin Cancer Res Phase 1b study of the novel anti-CXCR4 antibody ulocuplumab BMS in combination with lenalidomide plus low-dose dexamethasone, or with bortezomib plus dexamethasone in subjects with relapsed or refractory multiple myeloma. San Francisco, CA A phase I trial of intravenous CG, a replication-selective, prostate-specific antigen-targeted oncolytic adenovirus, for the treatment of hormone-refractory, metastatic prostate cancer.
Mol Ther 14 1: Clin Cancer Res 11 Cancer Blood BMC Cancer Clin Cancer Res 21 Adv Cancer Res Whole tumor antigen vaccines: Vaccines Basel 3 2: Targeting the tumor mutanome for personalized vaccination therapy. Oncoimmunology 1 5: Cancer vaccine adjuvants — recent clinical progress and future perspectives.
Login using
Immunopharmacol Immunotoxicol 37 1: Expert Opin Investig Drugs 18 7: Limacher JM, Quoix E. Oncolytic viruses for cancer therapy: Viruses 2 1: Moving oncolytic viruses into the clinic: Mol Ther Methods Clin Dev 3: Oncolytic viruses as immunotherapy: Onco Targets Ther 9: Cancer Sci Future Oncol 6 6: Transarterial injection of recombinant human type-5 adenovirus H in combination with transarterial chemoembolization TACE improves overall and progressive-free survival in unresectable hepatocellular carcinoma HCC.
Utilizing the BiTE bispecific T-cell engager platform for immunotherapy of cancer. Expert Opin Biol Ther 15 8: J Clin Oncol 34