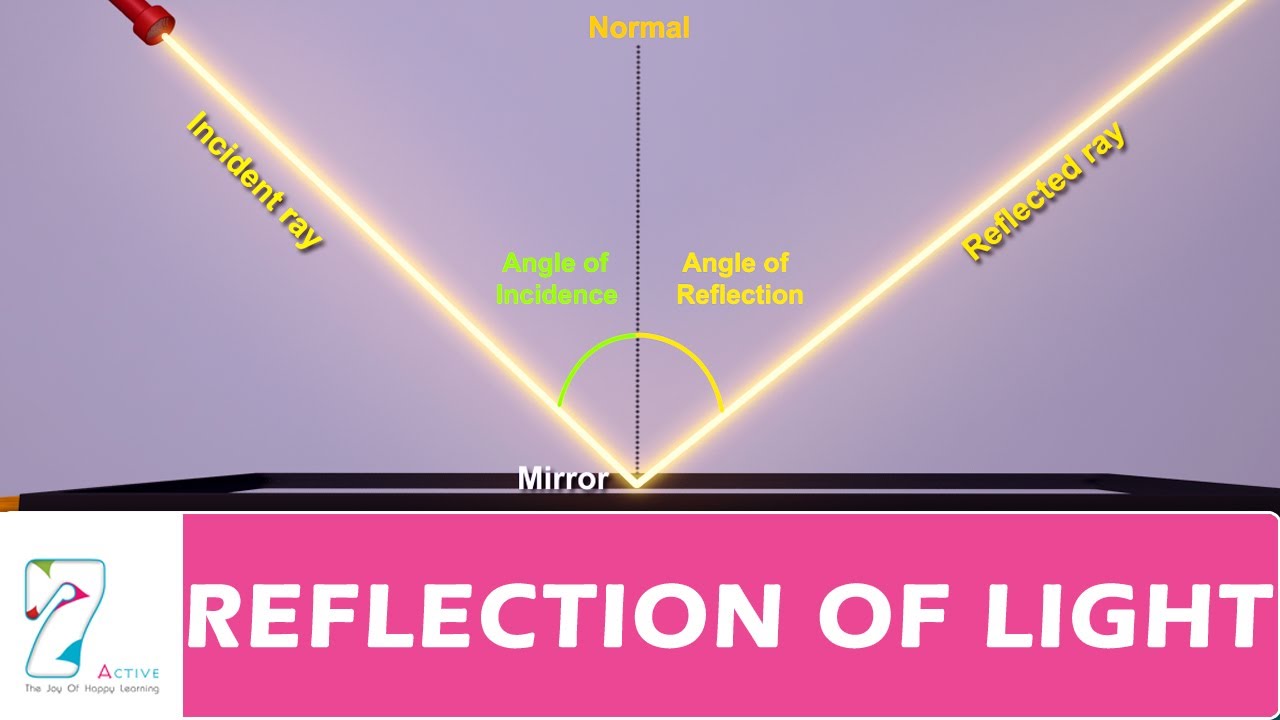
What is Light and How do We Explain it?
The Y axis is amplitude, and the X axis is distance in space. Electromagnetic waves consist of an oscillating electric field with a perpendicular oscillating magnetic field. More specifically, chemists study how different forms of electromagnetic radiation interact with atoms and molecules. Basic properties of waves: Amplitude, wavelength, and frequency. As you might already know, a wave has a trough lowest point and a crest highest point.
The Nature of Light
This is the property associated with the brightness, or intensity, of the wave. The horizontal distance between two consecutive troughs or crests is known as the wavelength of the wave. These lengths can be visualized as follows:. A two-dimensional representation of a wave. The amplitude is the distance from its central axis indicated by the red line to the tip of a crest. The wavelength is the distance from crest to crest, or from trough to trough.
The basic characteristics of a wave, including amplitude and wavelength. Keep in mind that some waves including electromagnetic waves also oscillate in space, and therefore they are oscillating at a given position as time passes. As you might imagine, wavelength and frequency are inversely proportional: This relationship is given by the following equation:.
The Nature of Light – The Physics Hypertextbook
Their product is the constant c c c , the speed of light, which is equal to 3. This relationship reflects an important fact: Calculating the wavelength of a light wave. A particular wave of electromagnetic radiation has a frequency of 1. What is the wavelength of this wave? We can start with our equation that relates frequency, wavelength, and the speed of light.
Next, we rearrange the equation to solve for wavelength. Lastly, we plug in our given values and solve. What would you expect to happen to the frequency of a light wave if its wavelength were increased by a factor of 1 0 10 1 0? Frequency and wavelength are inversely proportional. The last quantity we will consider is the period of a wave. The following table shows us this spectrum, which consists of all the types of electromagnetic radiation that exist in our universe. The electromagnetic spectrum is comprised of all the varieties of radiation in the universe. Gamma rays have the highest frequency, whereas radio waves have the lowest.
Visible light is approximately in the middle of the spectrum, and comprises a very small fraction of the overall spectrum. As we can see, the visible spectrum—that is, light that we can see with our eyes—makes up only a small fraction of the different types of radiation that exist. To the right of the visible spectrum, we find the types of energy that are lower in frequency and thus longer in wavelength than visible light.
These types of energy include infrared IR rays heat waves given off by thermal bodies , microwaves, and radio waves. These types of radiation surround us constantly, and are not harmful, because their frequencies are so low. To the left of the visible spectrum, we have ultraviolet UV rays, X-rays, and gamma rays. These types of radiation are harmful to living organisms, due to their extremely high frequencies and thus, high energies. It is for this reason that we wear suntan lotion at the beach to block the UV rays from the sun and why an X-ray technician will place a lead shield over us, in order to prevent the X-rays from penetrating anything other than the area of our body being imaged.
Gamma rays, being the highest in frequency and energy, are the most damaging. Luckily though, our atmosphere absorbs gamma rays from outer space, thereby protecting us from harm. Quantization of energy and the dual nature of light. We have already described how light travels through space as a wave. This has been well-known for quite some time; in fact, the Dutch physicist Christiaan Huygens first described the wave nature of light as far back as the late seventeenth century. For about 2 0 0 2 0 0 years after Huygens, physicists assumed that light waves and matter were quite distinct from one another.
According to classical physics, matter was composed of particles that had mass, and whose position in space could be known; light waves, on the other hand, were considered to have zero mass, and their position in space could not be determined. Because they were considered to be in different categories, scientists did not have a good understanding of how light and matter interacted. This all changed in 1 9 0 0 1 9 0 0 , however, when the physicist Max Planck began studying blackbodies — bodies heated until they began to glow.
- .
- Physics for Kids: Light Spectrum.
- .
- Light - Wikipedia;
- !
- .
- A God of Love!
Light from a light source located below the tube and stage of the microscope is initially unpolarized. This light first passes through the lower polarizer usually just called the polarizer , where it becomes polarized such that the light is vibrating from the users right to left. These directions are referred to as East right and West left. The light then passes through a hole in the rotatable stage of the microscope and enters the lower lens, called the objective lens.
Mounted within the microscope tube is a second polarizer, called the analyzer , that can be rotated or pushed so that in can be in the light path inserted position or not in the light path analyzer out position. The analyzer has a polarization direction exactly perpendicular to that of the lower polarizer These directions are usually referred to as North - South. If the analyzer is in, then the plane polarized light coming from the lower polarizer will be blocked, and no light will be transmitted though the ocular lens above.
If the analyzer is out, so that it is not in the light path, then the polarized light will be transmitted through the ocular lens. Next time we will see how this microscope is used to examine isotropic substances and determine their refractive indices. As discussed above, isotropic substance are those wherein the velocity of light or the refractive index does not vary with direction in the substance. Substances such as gases, liquids, glasses, and minerals that crystallize in the isometric crystal system are isotropic.
We here introduce the concept of the optical indicatrix then look at what we see when we look at isotropic substances through the polarizing microscope. We then see how to determine the refractive index of isotropic substances as a means to identify them, and then take a first look at uniaxial materials. The concept of the optical indicatrix is important as a visual means of looking at the way refractive index varies with direction in a substance. For isotropic minerals and substances the indicatrix is pretty trivial, since the refractive index does not vary with direction.
The optical indicatrix is simply a three dimensional object constructed by drawing vectors of length proportional to the refractive index for light vibrating parallel to the vector direction from a central point. The ends of all of the vectors are then connected to form the indicatrix. For isotropic minerals the indicatrix is a sphere as can be seen here. The indicatrix can be placed anywhere within or on a crystal so long as the crystallographic directions in the indicatrix are moved parallel to themselves.
Again, for the isotropic indicatrix, this is fairly trivial since the refractive indices do not correspond to crystallographic directions and the refractive indices are the same in all directions, but the usefulness of the indicatrix concept will become much more clear when we look at anisotropic substances. As discussed last time, the polarizing microscope has two polarizers. The lower polarizer often just called the polarizer is above the light source, and thus creates polarized light that vibrates in the East West direction.
The upper polarizer, called the analyzer, is polarized to create polarized light vibrating at 90 o to that produced by the lower polarizer. Thus, if there is only air, an isotropic substance, between the two polarizers, the E-W vibrating light is completely eliminated at the analyzer, and no light passes through the ocular lens. So, if we place a mineral grain on a glass slide glass is also isotropic , and view the grain through the ocular lens with the analyzer not inserted in the light path, we will be able to clearly see the grain. If the grain selectively absorbs light of certain wavelengths, then the grain will show its absorption color.
If we now insert the analyzer in the light path, the light coming out of the grain will still be polarized in an E-W direction, since isotropic substances do not change the polarization direction, and the analyzer will cut out all of this light. Thus, no light coming out of the mineral grain will pass through the analyzer. The mineral is thus said to be extinct in this position. Similarly, if we rotate the stage of the microscope, and thus rotate the grain, it will remain extinct for all rotation positions.
This is the primary means to determine whether or not a substance is isotropic.
Discussion
That is, rotate the grain on the microscope stage with the analyzer inserted. If the grain remains extinct throughout a o rotation of the stage, then the mineral or substance on the microscope stage is probably isotropic. Determination of Refractive Index for Isotropic Solids: In isotropic substances, there are only two optical properties that can be determined. One of these is the absorption color, as discussed above. The other is the refractive index. Tables of refractive indices for isotropic minerals, list only the refractive index for one wavelength of light.
Light: Electromagnetic waves, the electromagnetic spectrum and photons
The wavelength chosen is nm, which corresponds to a yellow color. Such a wavelength would be given off of a sodium vapor lamp. Since these are expensive and generate much heat, sodium vapor lamps are not generally used in optical mineralogy. Instead we use white light. Still, as we shall see later, we can determine the refractive index for nm.
Navigation menu
The comparison materials used are called refractive index oils. These are smelly organic oils that are calibrated over a range of refractive indices from 1. As you will see in lab, grains of the unknown substance are placed on a glass slide, a cover glass is placed over the grains, and a refractive index oil is introduced to completely surround the grains. This is called the immersion method. The grains are then observed with the analyzer not inserted.
If the grain has a refractive index that is very much different from the refractive index of the oil, then the grain boundaries will stand out strongly next to the surrounding oil. The grain will then be said to show high relief relative to the oil. High relief indicates that the refractive index of the grain is very much different from the refractive index of the oil. It does not tell us if the refractive index of the grain is less than or greater than the oil.
If the refractive index of the grain and the oil are closer, then the outline of the grain will not stand out as much from the oil. In this case, the grain is said to low relief relative to the oil. Again, low relief only indicates that the grain and oil have similar refractive indices, and does not indicate that the grain as a lower or higher refractive index than the oil. If the refractive index of the grain is exactly the same as the refractive index of the oil, the boundaries of the grain will not be visible. That is to say that the grain will completely disappear in the oil. In this case the grain is said to have no relief relative to the oil.
In order to determine whether the grain or the oil has a higher refractive index, a method called the Becke Line Method is used. A grain surrounded by oil when viewed through the microscope focused slightly above the position of sharpest focus will display two lines, one dark and one bright that concentric with the border of the grain. The brighter of these lines is called the Becke line and will always occur closest to the substance with a higher refractive index.
This can be used to determine if the grain or the oil has the higher refractive index. To use this method, one first focuses the microscope as sharply as possible on the grain of interest. It is also useful to use the iris diaphragm to cut down the incoming light as much as possible. This will make the Becke line stand out better. Then using the fine focus dial adjustment the microscope stage is lowered or the objective lens is raised slightly out of focus.
During this increase in focal distance one observes a moving bright Becke line. If the Becke line moves inward, the refractive index of the grain is greater than the refractive index of the oil. It is important to remember that the Becke line test is performed by increasing the distance between the grain and the objective lens. Thus, you should not memorize which way to turn the focusing knob, because it may be different with different brands of microscopes.
Note also that if the focal distance is decreased, rather than increased, then the opposite results will be obtained, that is with decreasing focal distance the bright Becke line will move into the substance with lower refractive index. For a grain with a refractive index less than that of the oil, the opposite effect will be observed. When raising the objective lens or lowering the stage so that the grain goes slightly out of focus, if the bright Becke line moves into the oil, then the oil has a refractive index greater than that of the grain.
This method can thus be used to start to narrow down the refractive index of the grain. For example, lets say that we first put grains of an unknown mineral in an oil with a refractive index of 1.
- Lesson Plan #4: Non-Fiction Text;
- Properties of Light.
- Introduction to electromagnetic waves.
- No More Running.
- Cuissons de base (Les indispensables t. 15) (French Edition).
- Eight things you might not know about light!
- ;
In this oil, let's say the Becke line test shows that the oil has a higher refractive index than the grain. We could then choose for our next test to put the unknown grains in an oil with a lower refractive index. If the relief is very high, then we would know to choose an oil with a much smaller refractive index. Thus, by performing several tests in several different oils, we could eventually find an oil that has a refractive index that exactly matches that of the grain.
In such a case the grain would have no relief relative to the oil and would thus disappear in the oil. But, this would not necessarily be true of the Becke lines. Recall from above that we said that refractive indices for grains and also oils are reported for a specific wavelength of light. That wavelength is nm, which corresponds to yellow.
Since we are using white light as an illuminator for our grain, the Becke line will be different for different wavelengths or colors of light. One other property we can determine for all minerals including anisotropic minerals is cleavage or fracture. This can be seen because it is usually necessary to crush or break minerals to obtain a size suitable for mounting in oils. This property can best be seen with the analyzer not inserted. Minerals or glasses that show concoidal fracture will have curved grain boundaries.
If a single cleavage direction is present, then it will show as parallel grain boundaries. Sometimes, the cleavages can be seen as breaks within a grain as well, although this is more common in thin sections than in grain mounts. Two cleavage directions will show as intersecting straight sided grain boundaries. Three or more cleavage directions should be visible as well, but it must be remembered that the microscope view is nearly 2-dimensional, so you may be able to see only 2 cleavage directions at once.
Summary of Observations of Isotropic Minerals Absorption color if present analyzer out Cleavage or fracture if present analyzer out Isotropic Character - with the analyzer inserted, the grain will be extinct during a complete o rotation of the microscope stage. An exact match will make the grain disappear in the oil and the Becke line test will show a orange - red Becke line moving into the grain and a blue - violet Becke line moving into the oil.
Next time we will look at the class of anisotropic minerals called uniaxial minerals. Examples of questions on this material that could be asked on an exam. Properties of Light and Examination of Isotropic Substances. The optical properties of crystals are, next to x-ray diffraction and direct chemical analyses, the most reliable properties available to distinguish and identify minerals.